Surfactants and microemulsions
A surfactant molecule is formed by two parts with different affinities for the solvents. One of them has affinity for water (polar solvents) and the other for oil (non-polar solvents). A little quantity of surfactant molecules rests upon the water-air interface and decreases the water surface tension value (the force per unit area needed to make available surface). That is why the surfactant name: "surface active agent".
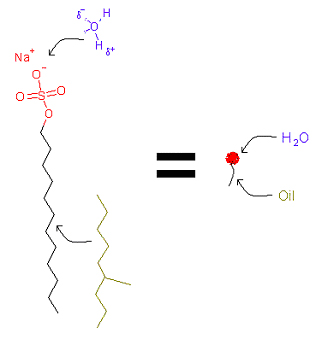
The polar "head" has affinity for water and the "tail" has affinity for oil.
When water, oil and a surfactant are mixed, the surfactant rests at the water-oil interface. These systems depending on their stability are called emulsions or microemulsions (thermodynamically stable). Although, the properties for an emulsion and a microemulsion are different, both obey the same principle: they try to form enough interface for preventing the polar non-polar solvent contact.
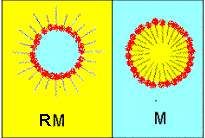
Microemulsions are very interesting systems, because the oil-surfactant-water interface forms a wide variety of structures to avoid the direct oil/water contact. The sizes of these structures are in the range of a few hundreds of nanometers, so the solutions are transparent. Micelles are the simplest structures: spherical or cylindrical objects formed by surfactant molecules, separating oil and water. Micelles are like drops of oil in water and reverse micelles are like drops of water in oil.
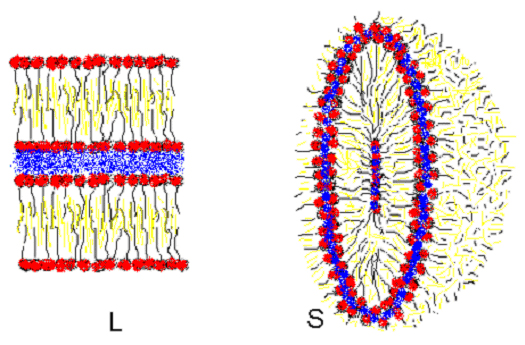
Another microemulsion structure is the lamellae: water and oil consecutive layers separated by surfactant layers conveniently oriented. The lamellae structure is similar to the smectic thermotropic phase, it presents birefringence and maintains the order even at diluted concentrations. This structure is related with the spherulite structure (onion structure). It is possible that spherulites are only out-of-equilibrium transient lamellar phases induced by mechanical work (yet to be proved) or by other stimulus. The thermodynamic stability of spherulites is still under study.
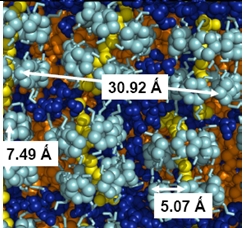
The bicontinuous structure or sponge phase is a quite intricate structure. As its name suggests, in this structure water and oil are continuous phases. The sponge structure is a good example: the sponge has a continuous structure, but it is possible to "fill" the sponge with a liquid. The liquid forms a continuous phase and the material of sponge forms also a continuous phase. Thinking that the sponge surface is the surfactant, we present a cartoon of a bicontinuous structure below:
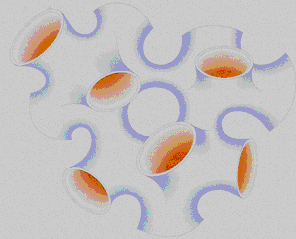
The "pipeline" forms an oil continuous phase (orange) and the exterior forms a water continuous phase (blue) [M. Daoud and C.E. Williams (Eds.); Soft Matter Physics, Springer-Verlag Berlin, Germany, 1999].
The Helfrich free energy (HFE), introduced in 1973 by W. Helfrich, explains the thermal interface fluctuations and topological interface changes involving the interface curvatures c1, c2 and the spontaneous interface curvature c0:
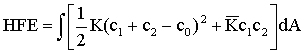
In the HFE equation, the K and 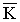 modulus are associated with thermal interface fluctuations and topological interface changes. For lamellar phases c1=c2=0 and only thermal fluctuations are possible. On the other hand, in the bicontinuous phase the saddle splay geometry fixes c1 = -c2, and the two terms of the HFE become energetically important at room temperatures.
modulus are associated with thermal interface fluctuations and topological interface changes. For lamellar phases c1=c2=0 and only thermal fluctuations are possible. On the other hand, in the bicontinuous phase the saddle splay geometry fixes c1 = -c2, and the two terms of the HFE become energetically important at room temperatures.
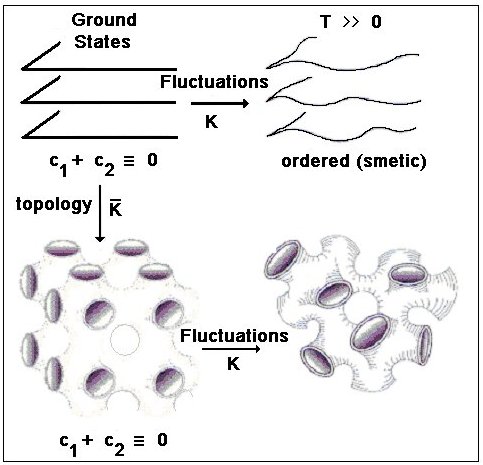
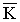 determine the interface thermal fluctuations and the interface topology respectively [M. Daoud and C.E. Williams (Eds.); Soft Matter Physics, Springer-Verlag Berlin, Germany, 1999] .
determine the interface thermal fluctuations and the interface topology respectively [M. Daoud and C.E. Williams (Eds.); Soft Matter Physics, Springer-Verlag Berlin, Germany, 1999] .Other microemulsion structures are possible: interconnected rod-like micelles, onions with an inner different structure, vesicles, etc. It has been found that the principal factors for explaining microemulsion structure changes are surfactant shape, entropic energy terms, as well as solvent properties as ionic force and pH.

 Overbeck/L2phase transition in the C
21monolayer
Overbeck/L2phase transition in the C
21monolayer