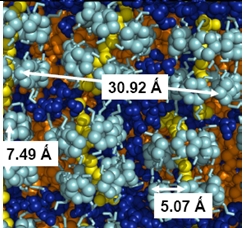
A Langmuir film is a one-molecule thick monolayer of amphiphilic water insoluble molecules located at the water/air interface. These systems are quasi-bidimensional and macroscopic because usually they are formed by at least 10 15 particles. They are studied because they present phases with varied forms of order, similar to the order in smectic liquid crystals. Also these systems are interesting to study 2-dimensional chemical systems and to prepare the Lagmuir-Blodgett films.
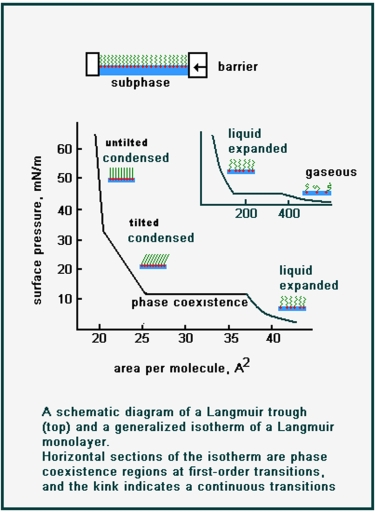
The Langmuir films are prepared on a computarized Nima LB trough, which can be seen in the figure 1. In this apparatus is where the insoluble surface active agent is deposited on the air water interface, generating a monolayer. The barriers compress the monolayer (reducing the area per particle A), at constant temperature T, and the Wilhelmy plate measures the lateral pressure, π = γ0 - γ (surface tension of the uncovered interface minus the covered interface surface tension). In this way, we obtain an isotherm, i.e. π(A, T), see figure 2. Along the compression the monolayer is observed with a Brewster angle microscope.
Procedure: prior deposition onto the water- air surface, we clean the surface sucking the surface, and then we observed the surface with the BAM to verify that there were no surface-active contaminants on it. A solution made with the surfactant and a volatile solvent is deposited with a syringe in the water interface at the working temperature. After some waiting time to allow solvent evaporation, the compression starts. All experiments are carried out in a dust-free environment.


 Overbeck/L2phase transition in the C
21monolayer
Overbeck/L2phase transition in the C
21monolayer